The Machines Amidst the Minestrone
When I lie on San Francisco’s Ocean Beach and focus my vision softly on the undulating water, I imagine the life that swarms underneath the ocean’s surface. The fish, the sharks, even the tiny happy phytoplankton, all bumping and grinding amongst one another like puckish tipsy high-schoolers. I imagine this happens all over the ocean, a teeming sea of water and fish and water again.
Of course, when I’m lying there, half-asleep and stubbornly refusing sunblock from my girlfriend, I’m thinking about it all wrong. Not just because fish don’t experience their puberty in the same way that humans do, but because I ignore the number of vastly different environments that make up the ocean. My bias is to think of landmarks as landmarks, but the ocean as essentially homogeneous.
Yet in reality there are looming underwater mountains and plunging ridges, hot water and cold, fast currents and lethargic ones. And neither is life distributed evenly. A phytoplankton bloom might inspire a chaotic feeding and mating orgy in one particular aquatic Vegas, while miles and miles of ocean are deserted, a remote Nevada highway on a Wednesday afternoon.
When we have difficulty seeing something, like the depths of the ocean, we tend to make incorrect assumptions about it. We reduce it, even caricature it, substituting cartoons for a more finely grained perception.
The ocean gave rise to cellular life, and despite hundreds of years of cell biology study, textbooks often still depict cells as some kind of watery soup. We have been observing cells closely since at least the 1670s, when Dutch lensmaker Antonie van Leeuwenhoek used a rudimentary microscope to describe the “animalcules” he saw in pond water. Our microscopy has continued to improve, with recent innovations breaking what were long thought to be fundamental limits in seeing the very smallest objects.
The UCSF lab of Dr. Ron Vale is among this cohort of powerful microscopists, showing the finer grain to the complex structures and movements within cells — the machines amidst the minestrone.
The closer we look, the more we are sure that cells are not in fact soup.
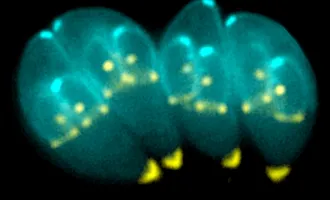
One surprising feature that has been observed by Vale’s lab is the presence of large clusters of signaling proteins inside of T cells. Recent work from a collaboration between Vale’s lab and that of Dr. Michael Rosen at UT Southwestern has shed light on this phenomenon.
T cells are the immune system quarterbacks responsible for activating an immune response. They are distinguished by a special kind of protein receptor on their cell surface, which, to keep things simple, are called T cell receptors. When the T cell receptor senses an imminent threat, an alarm signal marshals the T cell into action.
Cells use signaling networks to communicate information that they receive at the exterior of the cell to the interior, to the nucleus, which reacts to this information by deciding which genes to express and how intensely to express them. The canonical understanding of signaling is like the pony express: protein A activates protein B, which activates protein C, etc. But of course, it’s messier than that. Protein X might come in and turn off protein B before it can relay its message. A representation of all signaling networks in a cell looks something like a map of every airline flight in the world.
Complicating this further is the recent work from Vale’s Lab, led by postdoctoral fellow Xiaolei Su, and published in Science on April 7. They show a different strategy at play for T cell receptor signaling, one that may amplify signaling intensely — a PA system replacing whispers of gossip in the hallway.
When T cell receptors are activated, the signal is passed to a protein called LAT. But rather than simply relay the signal to another protein, dozens, if not hundreds, of copies of LAT cluster together with many copies of other proteins, including Grb2 and Sos1. Grb2 is known as a “scaffold” protein, since it binds the whole structure together. Su’s recent work, with an equal contribution from Jonathan Ditlev in the Rosen lab, shows how essential these scaffolding interactions are in the formation of gigantic LAT clusters.
Clustering allows the signal to be amplified because it reinforces the activation of the proteins within the cluster, like penguins protecting their warmth. While LAT is activated by a signal from the T cell receptor, other proteins inside of the cell have the ability to deactivate LAT and turn it off before it can pass on the signal (the aforementioned Protein X). Clustering protects LAT from these bullies by simply crowding the deactivators out.
The end result is a much stronger signal than may be accomplished without the clustering phenomenon. One thing T cells do when they are activated is divide over and over, increasing the pool of cells for fighting whatever infection. Su and Ditlev show that LAT clustering is essential for propagating the signal for proliferation. When they removed clustering ability, by changing the scaffolding domains of Grb2, they saw that cells were no longer activating the cell division pathway. In this way T cell clustering is essential for your immune system to function properly.
Interestingly, Su and Ditlev demonstrate that these protein clusters show liquid-like properties. Separate clusters can fuse seamlessly with one another, and proteins are constantly swirling around inside of any given cluster. The metaphors of scaffolding and architecture fall short of describing the reality. Proteins are simply different materials than brick, wood, or marble.
Likewise, proteins are not penguins, or quarterbacks, or high school bullies. Their functions are so diverse and dynamic that they resist easy classification. Perhaps “animalcules” — Leeuwenhoek’s term for pond microorganisms — could be re-appropriated, useful precisely because of its vagueness.
The more we look and the less we are forced to assume, the more we will know about how cells actually function. Biology is inextricably tied to microscopy. Metaphors allow us to imagine cellular activities, even to generate practical hypotheses, but seeing a cell in action is its own unimpeachable document.